Shapes of Covalent Molecules
Shapes of Covalent Molecules: Overview
This topic covers concepts, such as Hybridisation, Rules for Hybridisation, sp Hybridisation, sp2 Hybridisation, sp3 Hybridisation, sp3d Hybridisation, sp3d2 and d2sp3 Hybridisations & Hybrid Orbitals & Salient Features of Hybridisation etc.
Important Questions on Shapes of Covalent Molecules
In which of the following species, the underlined carbon atom is hybridised?
Which is the following has the regular tetrahedral structure?
The state of hybridisation of boron and oxygen atoms in boric acid molecule are respectively –
Hybridisation shown by in is:
The species having hybradisation is/are:
Identify the orbital hybridization at the two indicated carbons in the molecule below:
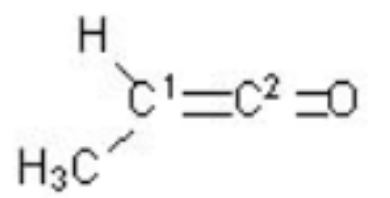
The molecule which contains and bonds in it is
In which of the following is in the hybridised state.
Match the following.
| Column - I (Compound of ) |
Column - II (Hybridization of ) |
||
| A) | I) | ||
| B) | II) | ||
| C) | III) | ||
| D) | IV) |
The correct match is
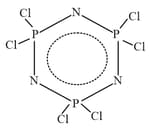
Find the ratio of and hybridised atoms which are present in ring in following molecule hexachlorotriphospazene
Which one of the following reactions involves change from to hybridisation of the central atom?
Which of the following statements is true about hybridization?
Bond is made by the overlap of which type of hybridized orbitals?
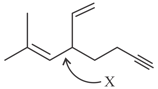
All carbon atoms are sp2 hybridised in
reacts with to form an adduct . The shape/geometry around the and ions, in the adduct, are respectively
The bond angle is present in
Phosphórous trichioride has a structure in which phosphorous is hybridization.
